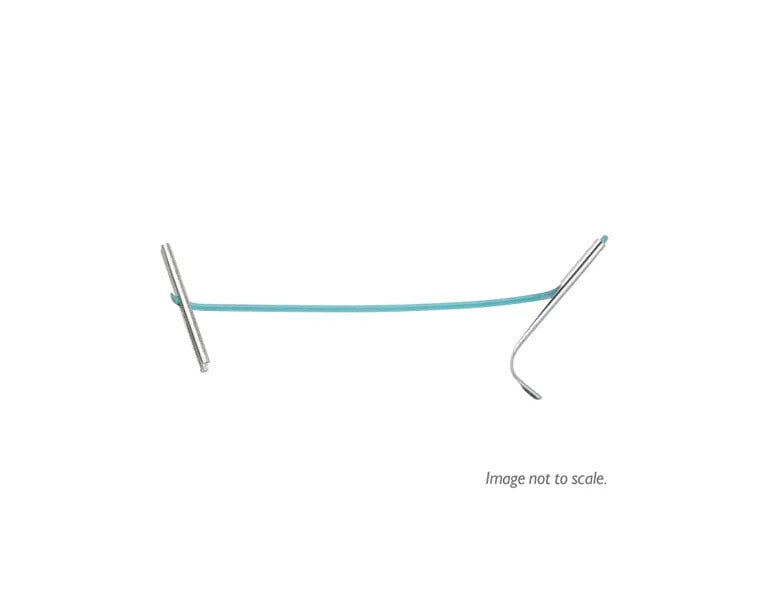
The UroLift™ System
The #1 chosen minimally invasive BPH procedure in the U.S.1 Treat a broad spectrum of anatomies with confidence.2,3
About the Procedure
The UroLift™ System is indicated for the treatment of symptoms due to urinary outflow obstruction secondary to benign prostatic hyperplasia (BPH), including lateral and median lobe hyperplasia, in men 45 years of age or older.
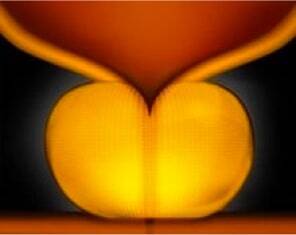
Enlarged Prostate
An enlarged prostate can narrow or even block the urethra.

Step 1
The UroLift™ Delivery Device is placed through the obstructed urethra to access the enlarged prostate.

Step 2
Small UroLift™ Implants are permanently placed to lift and hold the enlarged prostate tissue out of the way.

Step 3
The UroLift™ Delivery Device is removed, leaving an open urethra to improve flow and provide lasting BPH symptom relief.
Proven Mechanism of Action of the UroLift™ System
- Only BPH MIST with consistent durability in real world and clinical trials4,5
- Superior patient experience* among leading BPH procedures4,6-7
- An early treatment alternative to BPH medications5
- One of the most studied BPH MIST with 145+ peer-reviewed and clinical publications and 12,000 patients studied
Treatment with the UroLift™ System does not preclude future UroLift™ System treatments, TURP, or laser procedures.5
Patient Selection
The UroLift™ System provides consistent outcomes to a broad range of BPH patients.8
| Prostate Indications for the UroLift™ System✝ |
| Minimum prostate volume | None |
| Maximum prostate volume | 100 cc |
| Obstructive median lobe | Yes |
The UroLift™ System should not be used if the patient has:
- Prostate volume of >100 cc
- A urinary tract infection
- Urethral conditions that may prevent insertion of a delivery system into the bladder
- Urinary incontinence due to incompetent sphincter
- Current gross hematuria
UroLift™ Delivery Devices and Implants
The full line of UroLift™ products are designed for all prostate types.

Become a UroLift Physician
Learn about our onboarding process and path to long-term success.
Peer Perspectives
Learn about the latest clinical developments from your peers.
View Resources
Find helpful information on how to build the UroLift™ System into your practice and continuing education opportunities.
References
1. U.S. 2022 estimates based on US Market Model 2022-24 (5-17-22 FINAL), which is in part based on Symphony Health PatientSource® 2018-21, as is and with no representations/ warranties, including accuracy or completeness.
2. Rukstalis, Prostate Cancer Prostatic Dis 2018
3. UroLift System Instructions for Use
4. Kaplan, Prostate Cancer Prostatic Dis 2023
5. Roehrborn, Can J Urol 2017
6. Gratzke, BJU Int 2016
7. Tutrone, Can J Urol 2020
8. Eure, J Endourol 2019
9. Management estimate based on product sales as of January 2024. Data on file Teleflex Interventional Urology
*Patient experience defined as a combination of rapid symptom relief, low risk profile, and preservation of sexual function
† Food and Drug Administration (FDA) patient criteria for the UroLift™ System
+ Dr. Robert Cowles is a paid consultant of Teleflex
MAC12000-01 Rev B