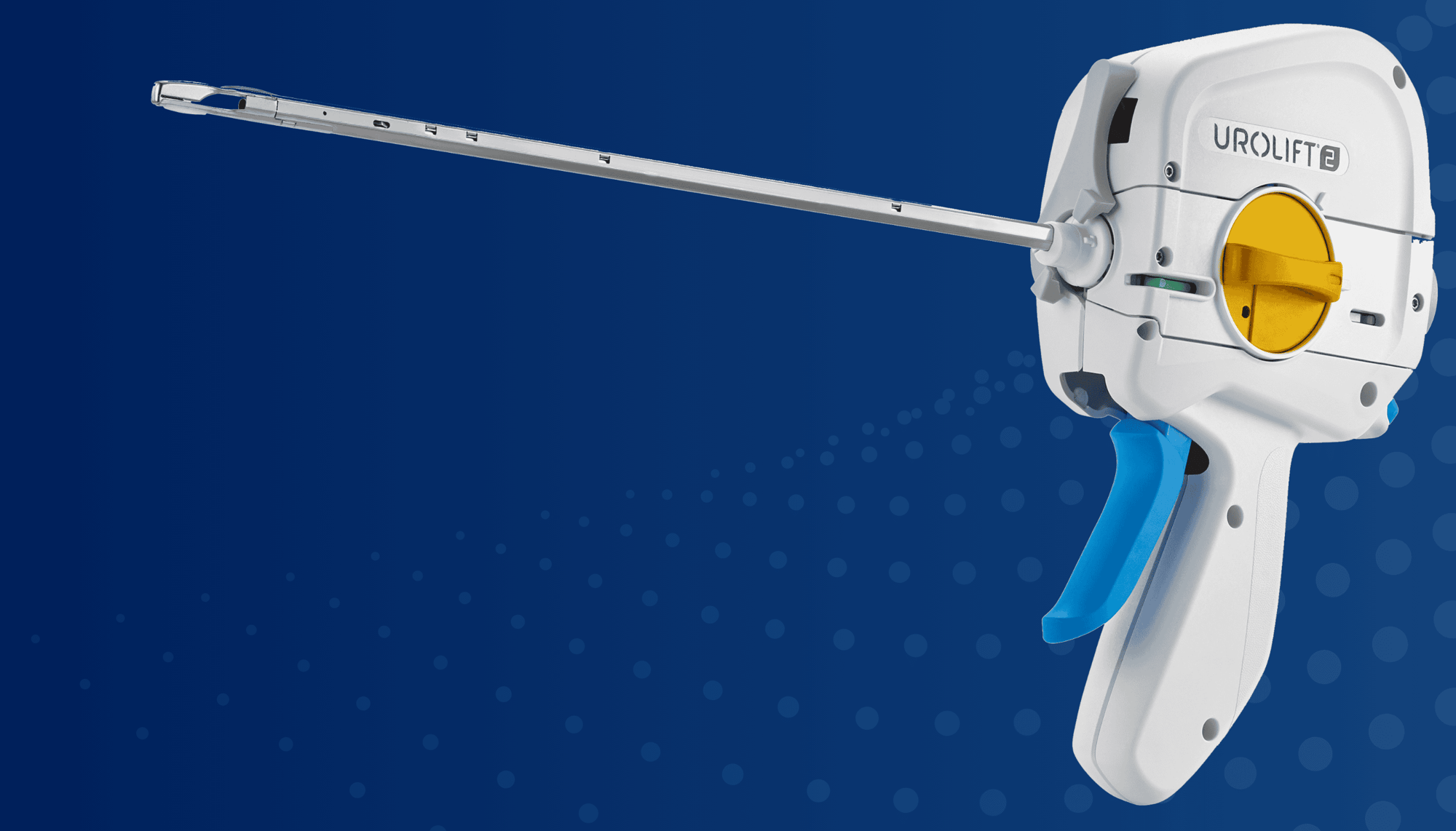
UroLift™ 2 System Portfolio
The #1 minimally invasive BPH procedure chosen by urologists in the U.S.1
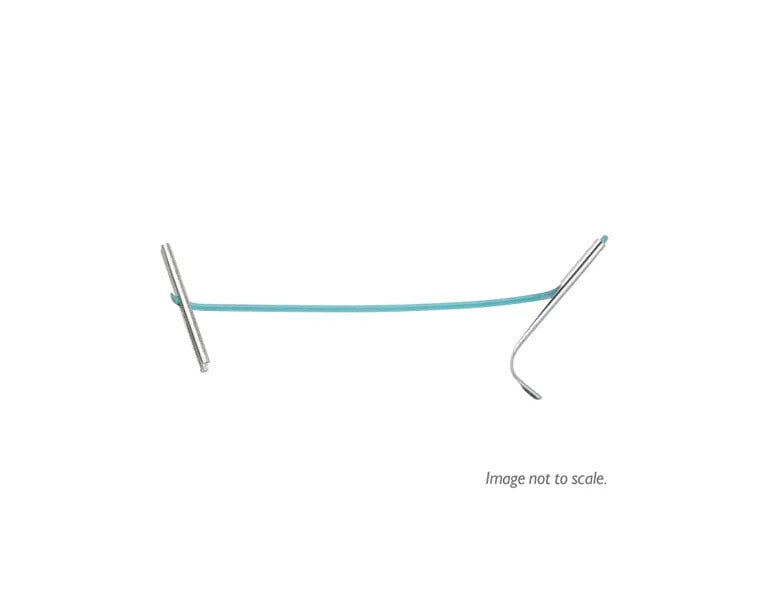
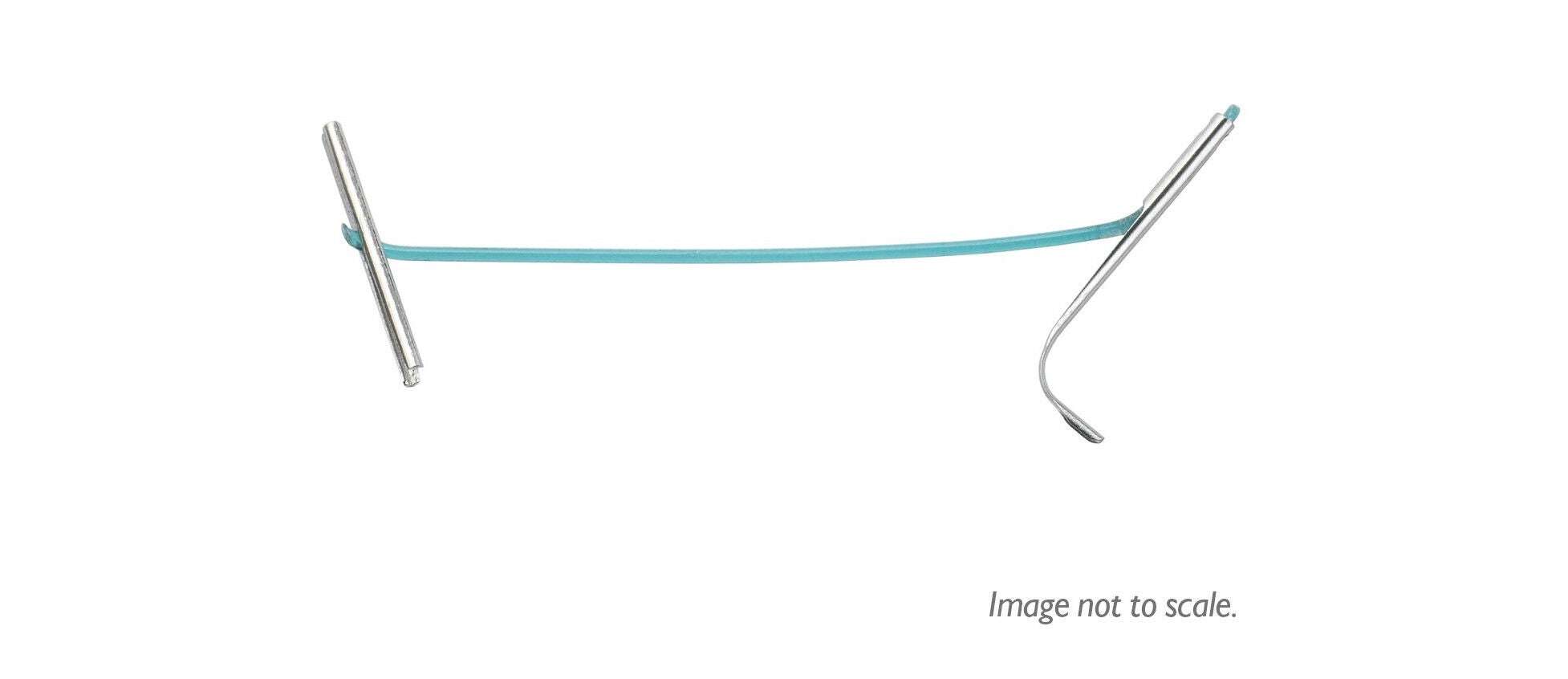
UroLift™ Delivery Devices and Implants
Designed for all prostate types.
UroLift 2 System
The unified UroLift™ 2 System platform is designed for the treatment of benign prostatic hyperplasia (BPH) symptoms in men with prostates types up to 100cc.2,3 As the most commonly performed minimally invasive BPH procedure in the U.S.,1 the UroLift System offers consistent durability in real world and clinical trials4,5 and superior patient experience* among leading BPH procedures.4,6-7
Streamlines the physician experience on a unified UroLift™ 2 System

Designed for more consistent and compressed implant deployment
Enhanced Confidence
Single ergonomic trigger, improved suture cutter,8 simplified troubleshooting tool, and fewer cystoscopic lens exchanges9
Innovative Delivery System
Typically one delivery handle per procedure, using an individual implant cartridge to deliver the UroLift™ System implant
Reduce Ecological Impact
60% less waste generated by one delivery handle per procedure, using individual implant cartridges to deliver the UroLift™ implant9
Proven UroLift™ Implant
No change to the UroLift™ implant components
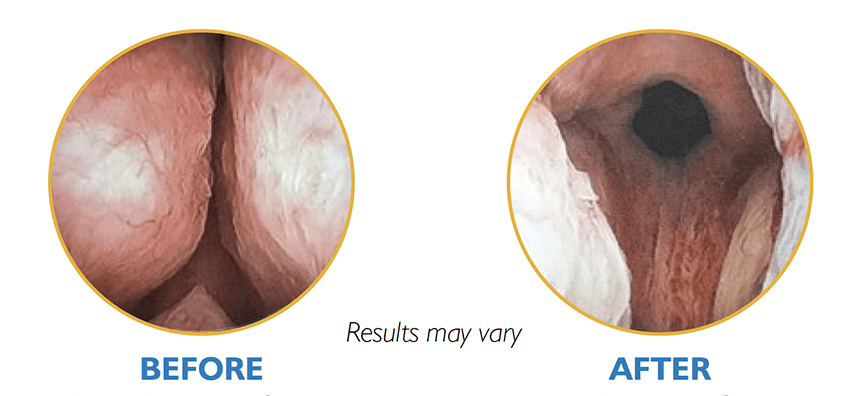
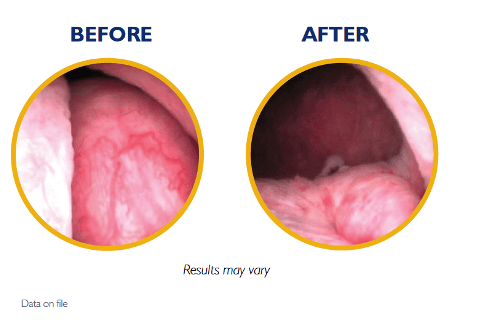
UroLift 2 System Advanced Tissue Control
Introducing the next generation of the UroLift System technology: the UroLift™ 2 ATC represents a breakthrough in BPH treatment. Its adaptable design enables physicians to customize treatments to each patient’s unique anatomy and allows a personalized approach to help maximize effectiveness.

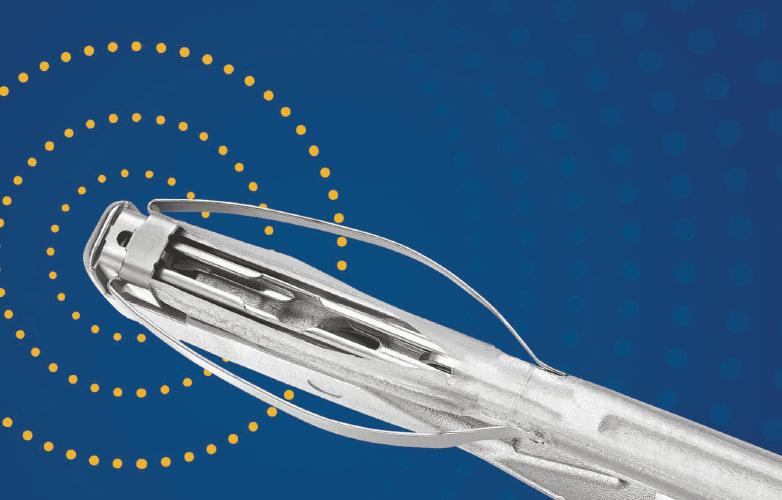
Unique tissue control wings hold tissue during manipulation and aid in visualization
Laser-etched needle location markers help with targeting and delivery of implants8
Rounded edges and flexible material designed to help reduce tissue trauma8
MRI Information
Non-clinical testing has demonstrated that the UroLift Implant is MR Conditional. A patient with this device can be safely scanned in an MR system immediately after placement meeting the following conditions:
- Static magnetic field of 3.0 Tesla or less
- Maximum spatial field gradient of 1,500 Gauss/cm (15 T/m) (extrapolated)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg for 15 minutes of continuous scanning (i.e., per pulse sequence) (First Level Controlled Operating Mode)
Under the scan conditions defined above, the UroLift Implant is expected to produce a maximum temperature rise of 2.4°C after 15 minutes of continuous scanning (i.e., per pulse sequence)
In non-clinical testing, the image artifact caused by the device extends approximately 15 mm from the UroLift Implant when imaged with a gradient echo pulse sequence and a 3.0 Tesla MRI system.
The safety of the delivery system has not been evaluated in the MR environment, and therefore, the implant deploying device should not be used within the MR environment.
Patient implant cards are provided to inform the patient that the UroLift implant is MR Conditional and can safely be scanned only under specific MR conditions. Download the printable version of our patient MRI card.
Warning: This device contains stainless steel and nitinol, an alloy of nickel and titanium. Persons with allergic reactions to these metals may suffer an allergic reaction to this implant. Prior to implantation, patients should be counseled on the materials contained in the device, as well as potential for allergy/hypersensitivity to these materials.
References
1. U.S. 2022 estimates based on US Market Model 2022-24 (5-17-22 FINAL), which is in part based on Symphony Health PatientSource® 2018-21, as is and with no representations/ warranties, including accuracy or completeness.
2. Rukstalis, Prostate Cancer Prostatic Dis 2018
3. UroLift System Instructions for Use
4. Kaplan, Prostate Cancer Prostatic Dis 2023
5. Roehrborn, Can J Urol 2017
6. Gratzke, BJU Int 2016
7. Tutrone, Can J Urol 2020
8. Data on file
9. Compared to prior-generation UroLift System. Data on file
* Patient experience defined as a combination of rapid symptom relief, low risk profile, and preservation of sexual function
MAC12001-07 Rev A